CXL News: Insurance Update

December 2017 Newsletter
December 19, 2017
Referral List Enrollment
December 22, 2017Here is an updated list of the 28 state & regional insurers that are currently covering CXL.
This list will constantly be updated, you can visit www.livingwithkeratoconus.com for the latest.
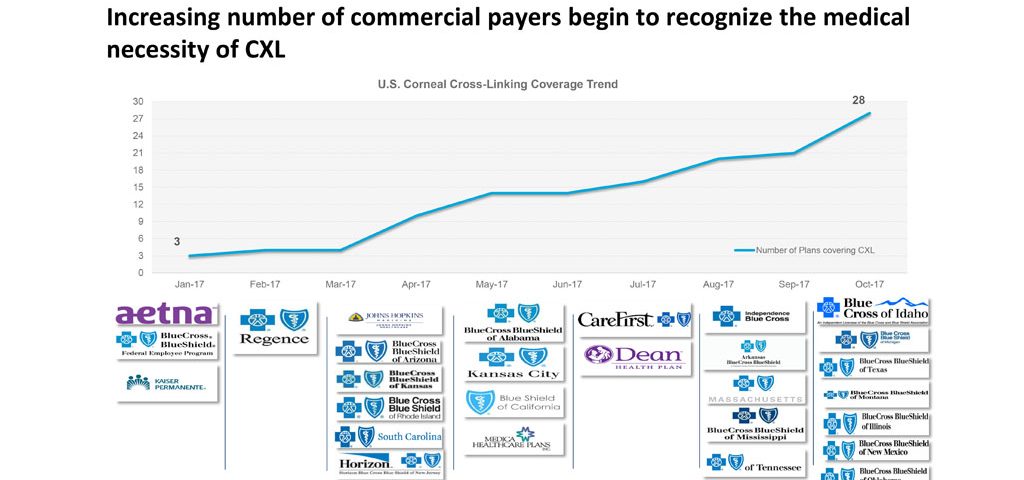
2017 concludes the first full year of corneal crosslinking (CXL) as an FDA approved treatment to halt the progression of keratoconus. Unfortunately, as many patients came to realize, FDA approval does not automatically confer insurance coverage. Many industry experts opined that it would take 3-4 years before payers would cover the costs associated with CXL. Instead, there has been remarkable movement on the part of many insurers during the first year of available treatment. The year ends with 28 state or regional insurers covering this treatment for progressive keratoconus.
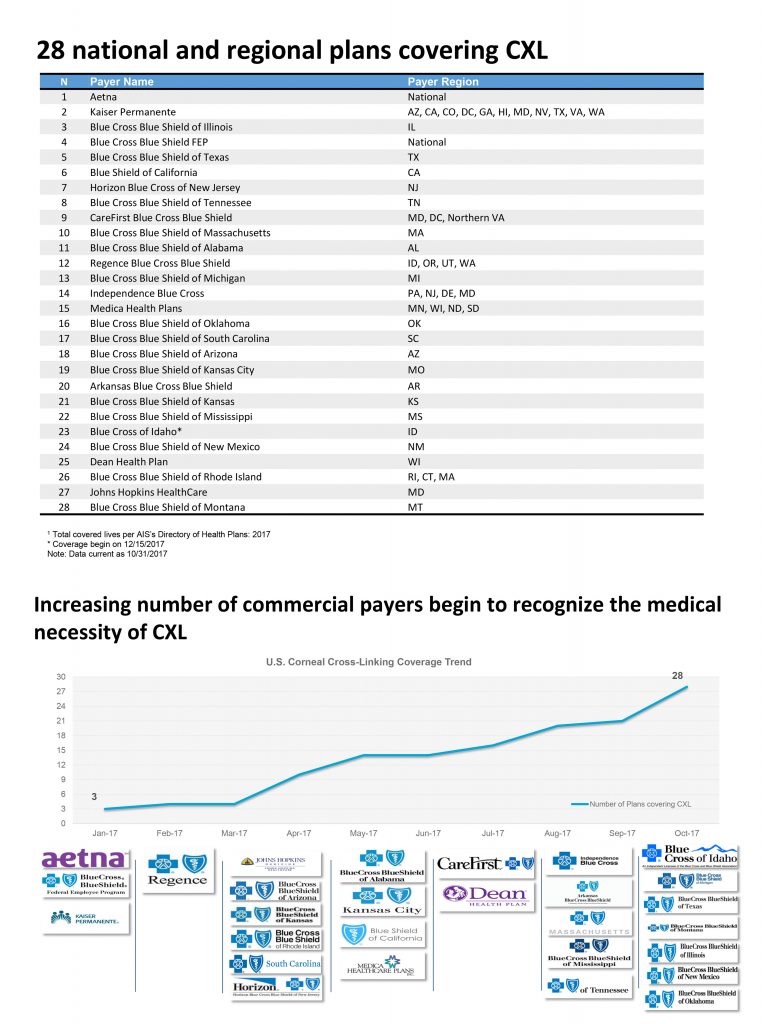
For an up-to-date list, visit www.livingwithkeratoconus.com.
It is important to read your insurance company’s policies regarding coverage of CXL. They might require documentation of disease progression, or certain diagnostic tests in order to qualify for coverage; many insurers will only cover the epi-off protocol.
Together you and your doctor should understand what is required in order for this to be a covered service.